Barbero Margherita
Aryl or Heteroaryl(indol-3-yl)methylium Salts as Electrophilic Reagents and as Lewis Acid Organocatalysts in Organic Syntheses
Carbocations are well-known highly reactive key intermediates. They are usually prepared in situ and immediately reacted, but some of them exhibit high stability which allows for easy isolation and use. Recently we reported the synthesis of bench-stable aryl or heteroaryl(indol-3-yl)methylium salts. Easily prepared in high yields and storable for long times without decomposition, they show great stability, also confirmed by their very low electrophilicity values.I
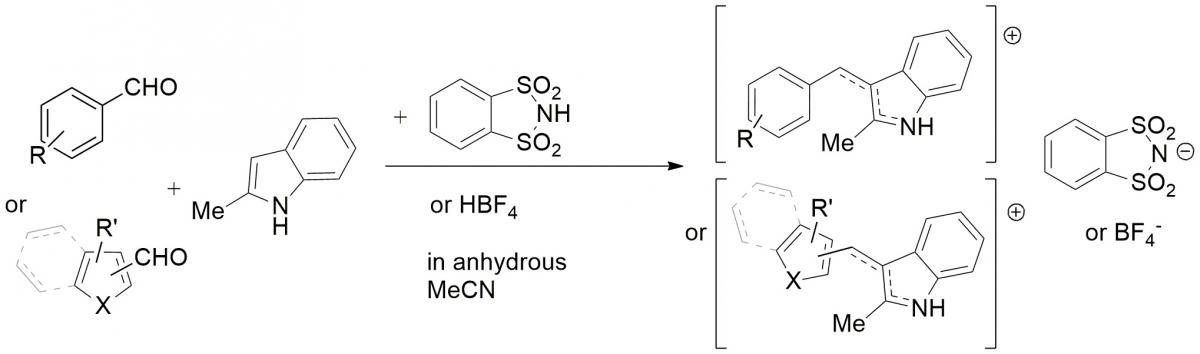
Until now, they have been explored as reagents in a direct organocatalyzed asymmetric alkylation of aldehydes and in the alkylation of silyl enol ethers of cyclic ketones, but research are undergoing in other use as electrophilic reagents with suitable nucleophiles.
Furthermore, carbocations have a low-lying empty pC orbital, which can accept electrons and therefore activate an electrophile towards nucleophilic attack as Lewis acids. After the first Mukaiyama’s reports on carbocation-catalyzed reactions, stable carbocations, such as tritylium ions, have been widely explored as organic Lewis acid catalysts. In these papers, tritylium salts were used as commercially available reagents or were generated in situ. As an alternative to them, we propose our air stable diarylmethylium salts as catalysts in common Lewis acids catalysts, such as dehydrative coupling and aldol, Michael and Diels-Alder reactions.
Tetrahedron Lett. 2016, 57, 4758-4762
Asian J. Org. Chem. 2015, 4, 337-245
J. Org. Chem. 2015, 80, 4791–4796
J. Org. Chem. 2012, 77, 4278-4287