Prandi Cristina - Blangetti Marco
Prof. Cristina Prandi
Dr. Marco Blangetti
Stefano Nejrotti
Simone Ghinato
Chiara Bellomo
Davide Arnodo
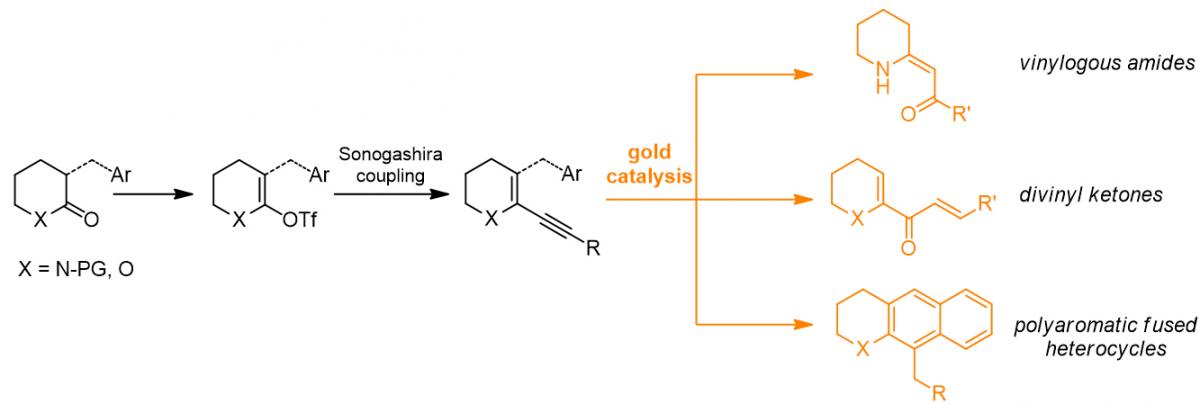
Synthesis of functionalized heterocyclic molecules through Au(I)-catalysed transformations of lactam-derived heterocycles. Heterocyclic rings containing N-, O- and S-atoms are a common motif in a wide variety of natural compounds, and thus the development of new methodologies for their functionalization is of particular interest for the synthesis of active molecules. In this context, homogeneous gold catalysis represents a powerful tool since it enables transformations which usually show very good selectivity, functional group tolerance, and require mild reactions conditions. Our research focused on the application of Au(I) catalysis to heterocyclic substrates derived from simple lactams, lactones and thiolactones, studying different methodologies for the synthesis of vinylogous amides, divinyl ketones and polyaromatic fused heterocycles. (collaboration with prof. Ernesto G. Occhiato, UniFi).
Selected publications:
- Eur. J. Org. Chem. 2017, 6228-6238
- Eur. J. Org. Chem. 2015, 3251-3265
- J. Org. Chem. 2013, 78, 11007-11016
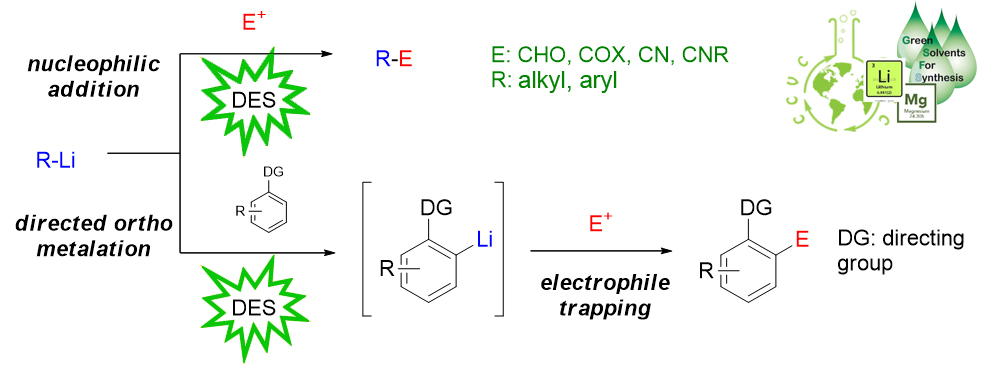
Reactivity of High Polar Organometallic Reagents in Deep Eutectic Solvents (DESs). The capacity to activate a specific C-H bond and transform it into a more versatile functional group represents an important and long-standing goal in chemistry. Highly polar organometallic reagents typically require aprotic VOCs, strictly anhydrous conditions and low temperatures owing to the notorious air- and moisture-sensitivity of s-block organometallic compounds. Recently, it has been reported the use of these reagents in nucleophilic addition to aromatic ketones, imines, nitriles and quinolines using highly polar solvents (DESs and water) at room temperature under open air conditions. Nowadays we are extending this methodology to other electrophilic functional groups (e.g amides, esters), and we are investigating the C-H bond activation of aromatic compounds by a directed ortho-metalation (DoM) approach using organolithium reagents in DESs as an alternative synthetic strategy for the preparation of versatile building blocks under green conditions.
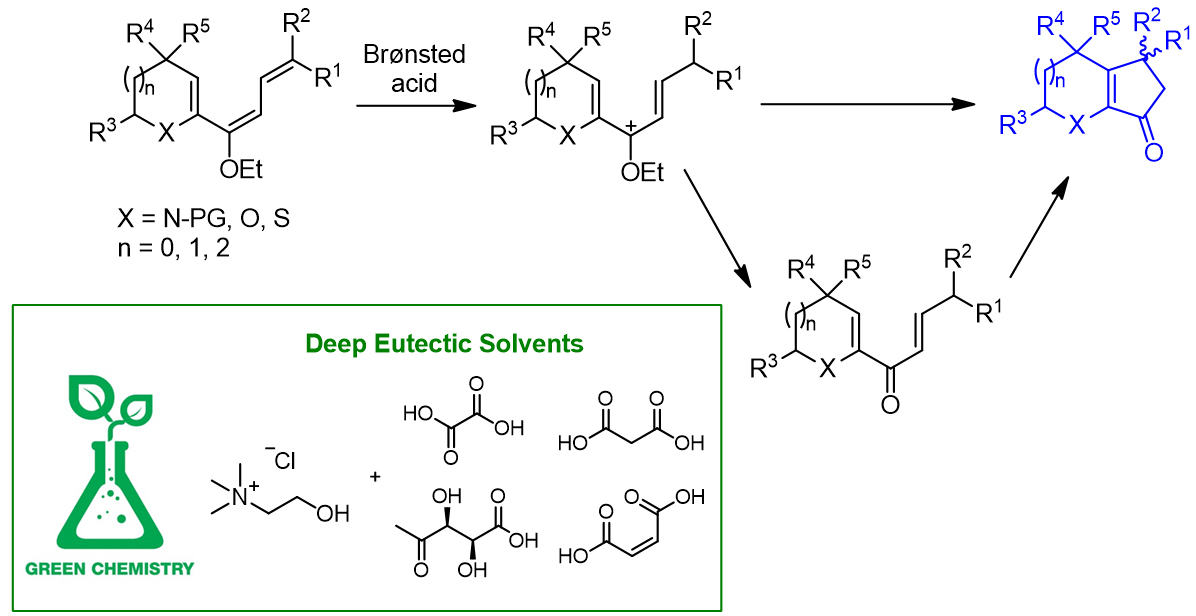
Advancements in the synthesis of highly functionalized cyclopentenones through the Nazarov electrocyclization. The Nazarov cyclization consists in an electrocyclic reaction of divinyl ketones to cyclopentenones, catalysed by Brønsted or Lewis acids. It represents one of the most straightforward synthetic ways to highly substituted cylopentenones, and in addition the stereochemistry of the products can be controlled by performing enantioselective versions of the cyclization. Beyond divinyl ketones, other classes of molecules can be employed as substrates for the Nazarov cyclization. This research line focused on the study of heterocyclic alkoxytrienes, which can easily undergo Nazarov cyclization to afford cyclopenta-fused heterocycles. The regio- and stereoselectivity of the reaction were studied in detail, both from an experimental and computational point of view. Recently, the interest moved towards the development of more sustainable conditions for the Nazarov cyclization, i.e. the use of green solvents such as deep eutectic solvents (DESs), which can have an active role in promoting the reaction themselves, thus acting as catalysts. (collaboration with prof. Ernesto G. Occhiato, UniFi)
Selected publications:
- Org. Lett. 2005, 70, 4542-4545
- Org. Lett. 2005, 7, 4345-4348
- J. Org. Chem. 2004, 69, 7704-7709
- J. Org. Chem. 2003, 68, 9728-9741
Synthesis of boron-containing photoluminescent materials for multimodal therapy applications. BODIPY (4,4-difluoro-4-bora-3a,4a-diaza-s-indacene) dyes have recently emerged as an interesting class of fluorescent taggers of carborane clusters for solid state luminescent devices and BNCT applications, thanks to their unique spectroscopic properties and their easiness of functionalization. Our research in this field, in collaboration of dr. Rosario Núñez of UAB, started with the synthesis of fluorescent carboranes-BODIPY/aza-BODIPY dyads by means of a facile Heck coupling reaction. These molecules are promising candidates for in vitro cell tracking applications, and nowadays our research is aimed at developing extended carborane-BODIPY dyads as multiresponsive agents for photodynamic therapy (PDT) applications.
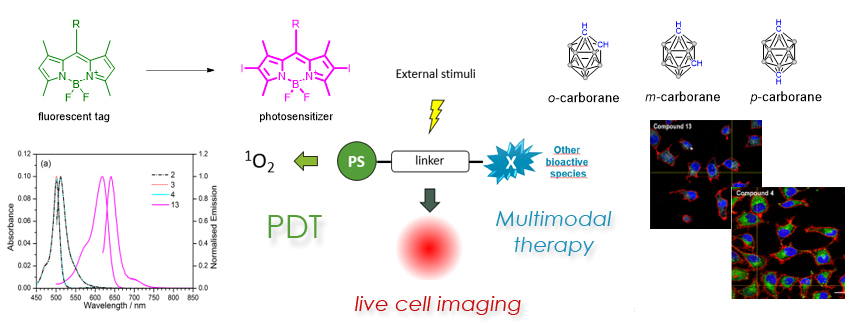
Selected Publications
- Chem. Eur. J. 2018, in press. doi:10.1002/chem.201802901
- Org. Biomol. Chem. 2017, 15, 884-893.
Synthesis of natural compounds for biological interest and bioimaging studies. Strigolactones (SLs) are a subject of increased scientific interest since 2008. Originally identified as allelochemicals involved in plant–parasite interactions, more recently SLs have been shown to play multiple key roles as signalling molecules, plant hormones and anticancer agents. For these reasons they have become a cutting-edge topic in plant biology and agronomy, and have great potential in modern agriculture for regulation of plant development and interactions. The research interest of our group in SLs chemistry is mainly focused on the synthesis of new structural analogues with high bioactivity, on the development of fluorescent-tagged derivatives for bioimaging purposes and on the investigation of the anticancer activity of natural and synthetic compounds belonging to the SL class.
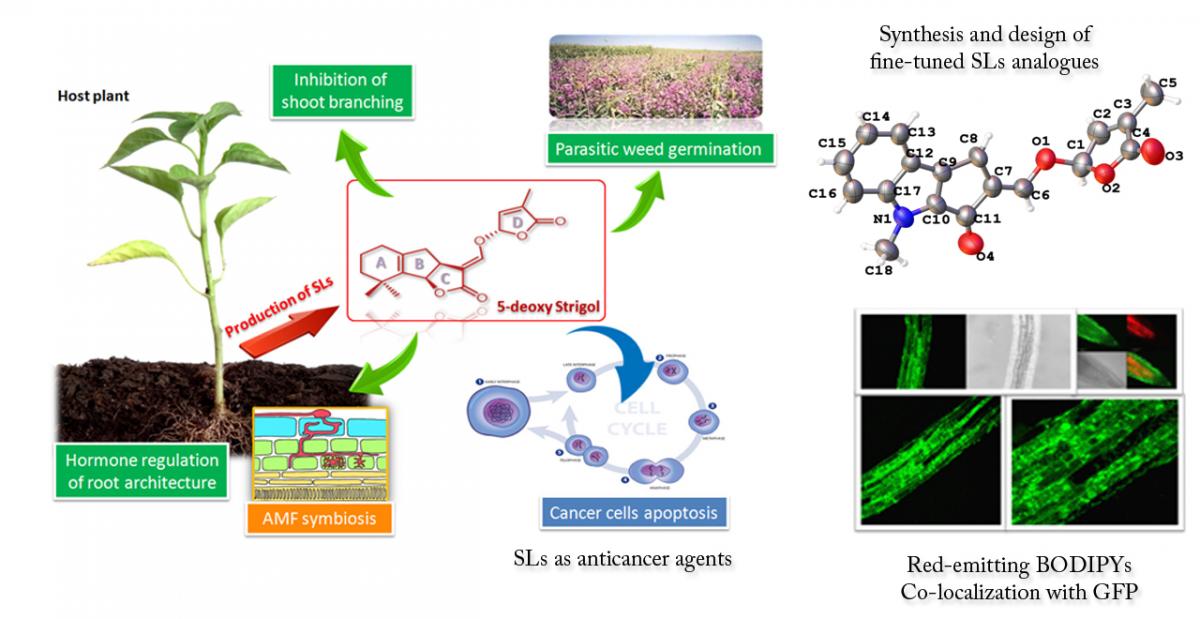
Selected publications:
- Org. Biomol. Chem. 2017, 15, 8218-8231.
- Mol. Plant 2016, 9, 1099-1118.
- Curr. Genet. 2017, 63, 201-213
- Pest Manag. Sci. 2016, 72, 2026-2034.
- Mol. Plant 2015, 8, 1809-1812.
- Cancer Biol. Ther. 2015, 16, 1682-1688.
- Org. Biomol. Chem. 2014, 12, 2960-2968.
- Mol. Plant 2013, 6, 141-152.
- Mol. Plant 2013, 6, 113-127.
- Breast Cancer Res. Tr. 2012, 134, 1041-1055.
- Org. Biomol. Chem. 2009, 7, 3413-3420.
Highly functionalized unsaturated systems in superbasic medium. In the past years our research has been focused on exploiting the synthetic usefulness of metalated alkoxy-1,3-dienes as building blocks in organic synthesis. The treatment of α,β-unsaturated acetals in the presence of mixed organoalkali-metallic reagents affords the corresponding alkoxydienes, which can be further metalated in situ and quenched with several electrophiles. Depending on the nature of the quencher, the alkoxydienylic scaffold has been exploited a) for the synthesis of heterocyclic systems, b) in highly diastereoselective reactions with chiral electrophilic reagents, and c) as alternative synthon for the carbonylative Pd-catalyzed Suzuki reactions in the synthesis of biological relevant compounds.
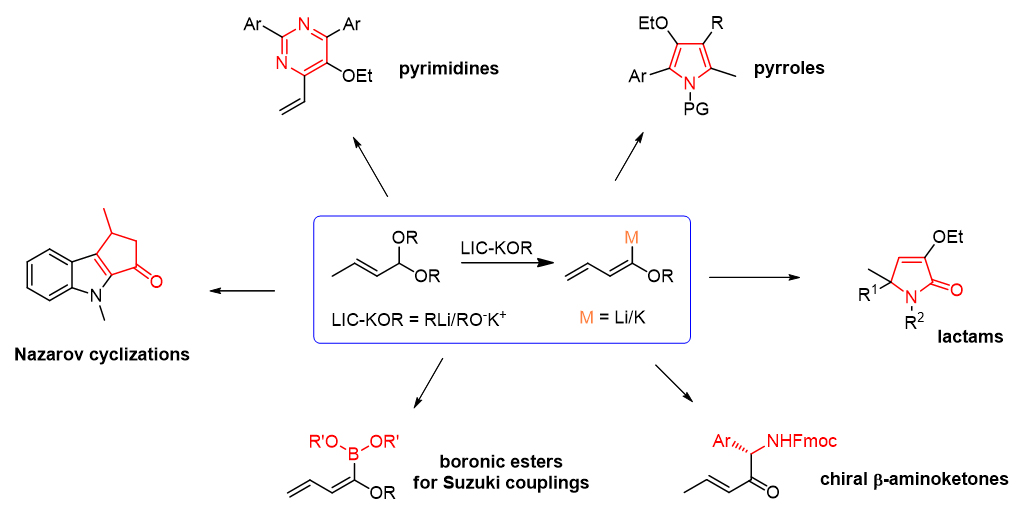
Selected publications:
- Molecules 2013, 18, 1188-1213.
- J. Org. Chem. 2011, 76, 1814-1820.
- Org. Biomol. Chem. 2011, 9, 2535-2538.
- Org. Lett. 2009, 11, 3914-3917.
- Chem. Commun. 2008, 1689-1691.
- Eur. J. Org. Chem. 2007, 5867-5874.
- Eur. J. Org. Chem. 2007, 1318-1323.
- Eur. J. Org. Chem. 2006, 3451-3456.
- Eur. J. Org. Chem. 2006, 2463-2483.
- J. Org. Chem. 2002, 67, 7144-7146.