Deagostino Annamaria and Renzi Polyssena
Annamaria Deagostino
Polyssena Renzi
Alberto Lanfranco
Jacopo Scarfiello
Marco Rusconi
Eugenia Magi
Synthesis of theranostic antitumoral agents for BNCT/MRI applications. This research line has been developed in strict collaboration with prof. S. Geninatti-Crich. In the last years, the group of Prof. Deagostino optimised the synthesis of several lipophilic GdMRI/BNCT agents. BNCT is a binary radiation therapy used for the treatment of tumours. The molecules synthesised can be classified as “theranostic agents” since contain a carborane cage for BNCT applications and a Gd-DOTA complex which is an MRI probe and a GdNCT agent and allows the analysis of agent biodistribution essential for a precise design of the therapy. All these agents have been tested in vitro and one in vivo showing a good efficacy for the treatment of several tumours, e. g. lung tumour and mesothelioma. More recently, the research has been focused on boronated curcumine derivatives for the treatment of Alzheimer’s disease (in collaboration with prof. Simonetta Geninatti Crich).
Selected Publications
- Eur. J. Med. Chem. 2024, 270, 116334.
- Scientific Reports 2023, 13 (1), 620.
- Org. Biomol. Chem. 2022, 20, 5342.
- J. Contr. Rel. 2018, 280, 31.
- Chemmedchem 2017, 12, 502.
- Future Med. Chem. 2016, 8, 899.
- Org. Biomol. Chem. 2015, 13, 3288.
- Nanomedicine 2015, 11, 741.
- Org. Biomol. Chem. 2014, 12, 2457.
- Chem. Eur. J. 2013, 19, 720.
- Anti-Cancer Agents Med. Chemistry 2012, 12, 543.
- Chem. Eur. J. 2011, 8479.
- Org. Biomol. Chem. 2008, 6, 4460.
Visible light driven transformation of unsaturated compounds. In recent years, photocatalysis has assumed a central role, enabling the generation of unique reactivity through processes that are not feasible through traditional thermal transformations. In this context, our research group has focused its attention on the photoinduced formation of key sulfur and nitrogen-centered radicals with the aim of utilizing them for the functionalization of unsaturated compounds. By employing innovative methodologies, we have identified novel approaches for the synthesis of sulfones, thioethers, functionalized pyrrolidines and tetrahydropyridazine under mild reaction conditions using visible light.
Selected Publications
- Adv. Synth. Catal. 2023, 365, 4623. Featured in Org. Chem. Highlights 2024, May 27.
- Chem. Sci. 2023, 14, 2721.
- J. Org. Chem. 2023, 88, 6420. JOC Special Issue Progress in Photocatalysis for Organic Chemistry.
- Org. Chem. Front., 2022, 9, 906; (cover picture).
- J. Org. Chem., 2021, 86, 3300.
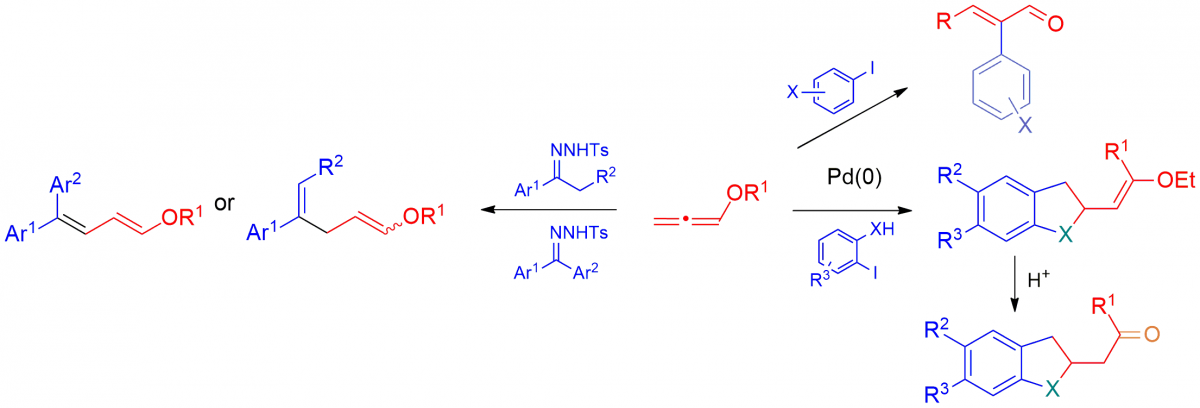
New methodologies for the preparation of highly functionalised molecules catalysed by Pd(0) starting from allenes and conjugated dienes, also in a domino fashion. 1,2-Dienes and 1,3-dienes are interesting substrates because of their high reactivity. In fact, when they undergo a carbopalladation process a π-allyl palladium intermediate is formed and at least two reactive pathways have to be considered. If a nucleophile is present in the reaction medium the addition product is obtained, otherwise a β-H elimination occurs. The reactivity of 1-alkoxy-π-allylpalladium complexes, obtained from the corresponding alkoxydienes, was explored, and the dramatic effect of the alkoxy group on the regioselectivity of the Pd(0) catalysed coupling reactions was demonstrated. New methodologies for preparing α-arylated α,β-unsaturated aldehydes, substituted 3-alkenylindoles, 2-alkoxy-3-alkylidene-2,3-dihydrobenzofuranes and -indolidines were reported. More recently, N-Tosylhydrazone addition to Pd(II)- π-allyl complexes has been studied for the regio- and stereoselective synthesis of conjugated and skipped dienes.
Selected Publications
- Org. Lett. 2018, 20, 6891.
- Chem. Eur. J., 2018, 24, 5484.
- Tetrahedron Lett. 2015, 56, 5791.
- Eur. J. Org. Chem. 2013, 2013, 6990.
- Curr. Org. Chem. 2011, 2390.
- Curr. Org. Chem. 2010, 14, 230.
- Org. Biomol. Chem. 2010, 8, 2020.
- Tetrahedron 2008, 64, 10344.
- Org. Lett. 2003, 5, 3815.